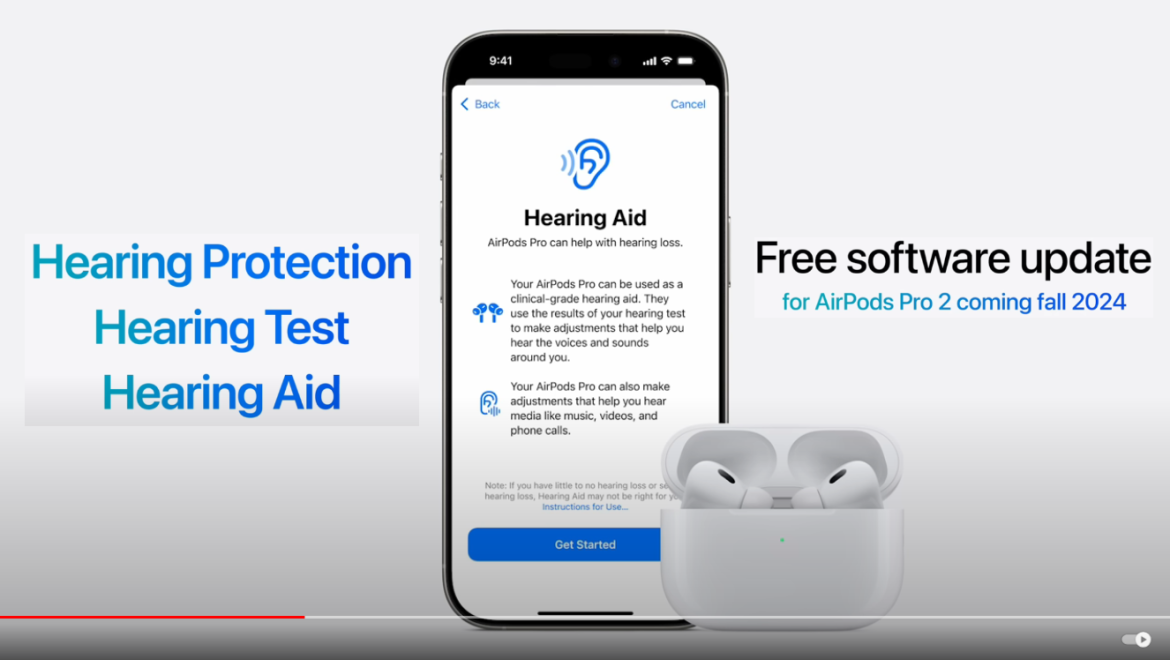
In a landmark decision, the U.S. Food and Drug Administration (FDA) has authorized the first-ever over-the-counter (OTC) hearing aid software device, marking a significant advancement in accessibility to hearing assistance technology. The software, called “Hearing Aid Feature” (HAF), is designed to work with compatible versions of Apple’s AirPods Pro headphones, effectively transforming them into OTC hearing aids.
Revolutionary Technology
The HAF utilizes a self-fitting strategy that allows users to customize their hearing experience without the need for professional assistance. Once installed and tailored to the individual’s hearing needs, the software enables compatible AirPods Pro to amplify sounds for adults aged 18 and older who experience mild to moderate hearing impairment.Dr. Michelle Tarver, acting director of the FDA’s Center for Devices and Radiological Health, emphasizes the importance of this authorization: “Today’s marketing authorization of an over-the-counter hearing aid software on a widely used consumer audio product is another step that advances the availability, accessibility, and acceptability of hearing support for adults with perceived mild to moderate hearing loss.”
The FDA reports that more than 30 million American adults experience some degree of hearing loss. This condition can significantly impact communication, relationships, work performance, and emotional well-being. The new software aims to provide a more accessible and potentially more affordable option for those who might benefit from hearing assistance but have hesitated to pursue traditional hearing aids.
Clinical Validation
Apple conducted a comprehensive clinical study involving 118 subjects across multiple U.S. sites to evaluate the effectiveness of the HAF. The results demonstrated that users of the self-fitting strategy achieved similar perceived benefits as those who received professional fitting of the same device. Moreover, the study showed comparable performance in tests measuring amplification levels in the ear canal and speech understanding in noisy environments.
User Experience and Customization
The HAF is set up using an iOS device, such as an iPhone or iPad. It accesses the user’s hearing levels from the iOS HealthKit to customize the experience. Users can further refine volume, tone, and balance settings after the initial setup, providing a personalized hearing solution.
Broader Implications
This authorization aligns with the FDA’s 2022 regulations that established the OTC hearing aid category, allowing adults with perceived mild to moderate hearing loss to purchase hearing aids directly from stores or online retailers without the need for a medical exam, prescription, or audiologist visit.The approval of the HAF not only represents a technological breakthrough but also signifies a shift in how hearing assistance can be delivered to millions of Americans. It has the potential to reduce barriers to entry for those seeking hearing support, potentially leading to earlier intervention and better long-term health outcomes.As Apple prepares to roll out this feature in more than 150 countries and regions, including the U.S., Europe, and Japan, the medical community and consumers alike are eagerly anticipating the impact this innovative approach will have on hearing health management.